
Neurodegenerative disorders like Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD) are typically distinguished by their hallmark clinical features—memory loss in Alzheimer’s, motor difficulties in Parkinson’s, and so on. But what if we shifted our understanding to see these disorders not just as isolated conditions but as part of a broader picture of impaired energy metabolism? This shift could help us understand the root causes of these diseases more effectively and open the door to novel therapeutic approaches. In their recent article in Translational Neurodegeneration, Matthew C. L. Phillips and Martin Picard offer such a perspective by conceptualizing neurodegenerative disorders as “metabolic icebergs.”
The Metabolic Iceberg: What Lies Beneath
Phillips and Picard propose a framework that likens neurodegenerative disorders to “metabolic icebergs.” In this analogy, the visible tip of the iceberg represents the hallmark symptoms and protein aggregates that clinicians use to diagnose diseases like AD, PD, ALS, and HD. These are the most apparent and recognizable manifestations of the conditions: memory decline, movement difficulties, muscle weakness, and psychiatric symptoms.
However, beneath the surface lies the bulk of the iceberg, which includes impaired mitochondrial biology affecting tissues throughout the body. Mitochondria, often called the powerhouses of cells, are responsible for generating energy, regulating metabolism, and managing cellular stress. Phillips and Picard emphasize that dysfunctional mitochondria are at the root of neurodegenerative disorders, not just in the brain but throughout multiple body
systems. This broader view explains why symptoms often go beyond the nervous system, such as the cardiovascular and muscular issues seen in these diseases.
Finally, the base of the iceberg encompasses the underlying factors driving mitochondrial dysfunction. These factors include genetic mutations linked to familial forms of the diseases, but also—crucially—environmental influences. Modern industrial toxins, unhealthy dietary patterns, chronic stress, and lack of physical activity all contribute to impaired mitochondrial health and, over time, lead to the visible symptoms of neurodegeneration.
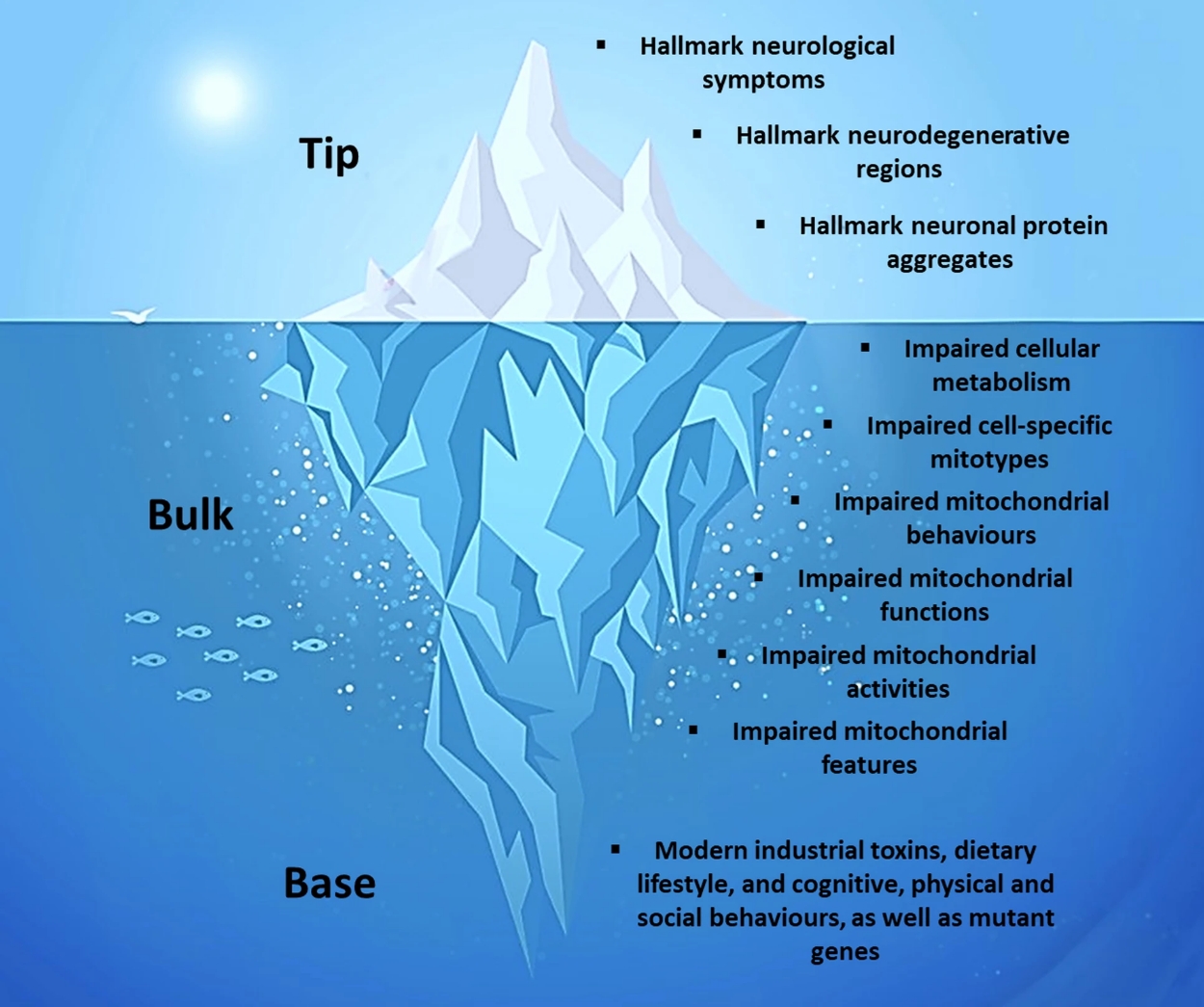 Fig. 3: From: Neurodegenerative disorders, metabolic icebergs, and mitohormesis
Fig. 3: From: Neurodegenerative disorders, metabolic icebergs, and mitohormesis
Splitting vs. Lumping: A New Way of Classifying Neurodegenerative Disorders
Historically, the medical field has adopted a “splitting” approach to neurodegenerative disorders—each condition is treated as a separate entity based on its unique clinical, anatomical, and pathological characteristics. This method allows for more precise diagnosis and symptom management. However, Phillips and Picard argue that this approach has significant limitations. It overlooks the broader, underlying dysfunction that drives the various disorders and leads to treatment strategies that focus mainly on suppressing symptoms, rather than addressing their root
causes.
Instead, Phillips and Picard suggest a “lumping” approach, grouping these diseases by their shared feature of mitochondrial impairment. By adopting this perspective, we can begin to view neurodegenerative disorders as multisystemic conditions needing comprehensive, restorative therapies rather than narrowly focused, symptom-suppressive interventions.
Activating Mitohormesis: A Path to Prevention and Treatment
The concept of mitohormesis emerges as a critical therapeutic opportunity in this framework. Mitohormesis refers to a biological process where mild mitochondrial stress triggers an adaptive response, ultimately strengthening the body’s ability to cope with stress. It’s like the idea of “what doesn’t kill you makes you stronger”—in small, controlled doses, stress can lead to resilience.
Phillips and Picard propose that mitohormesis can be activated by implementing a balanced oscillation between mitochondrial stress and recovery phases. For example, challenging the mitochondria through physical exercise, intermittent fasting, and even exposure to certain environmental stressors (like cold or heat) can stimulate mitochondrial repair and adaptation, as long as sufficient recovery time is allowed between challenges. This balanced approach can recalibrate impaired mitochondrial biology, potentially offering a powerful preventative and therapeutic strategy for those at risk of, or already experiencing, neurodegenerative disorders.
The Role of Modern Lifestyle Factors
Environmental factors at the base of the metabolic iceberg are largely modifiable, and this is where the greatest opportunity for intervention lies. The authors emphasize that our modern lifestyle—characterized by chronic exposure to industrial toxins, diets high in processed carbohydrates, and lack of physical and cognitive challenges—creates the perfect storm for mitochondrial dysfunction.
Reducing exposure to toxins like heavy metals and air pollutants, adopting a nutrient-dense, low- carbohydrate diet, and integrating regular exercise and cognitive stimulation into daily routines are all ways to enhance mitochondrial function and reduce the risk of neurodegeneration. These lifestyle changes are not merely preventive; they can also act as therapeutic tools to activate mitohormesis and promote mitochondrial recovery in individuals already dealing with these diseases.
A Call for a Broader View of Neurodegeneration
The metabolic iceberg framework encourages us to look beyond the visible symptoms of neurodegenerative disorders and address the deeper, often hidden causes of these diseases. Rather than seeing AD, PD, ALS, and HD as distinct conditions requiring separate treatments, Phillips and Picard suggest we should consider their common denominator—impaired mitochondrial function—and focus on restorative, system-wide therapies.
This perspective aligns well with the brain energy theory, which emphasizes the importance of metabolic health for maintaining optimal brain function. By understanding neurodegenerative disorders as disorders of impaired energy metabolism, we can develop more effective prevention and treatment strategies that address the root of the problem, rather than just the symptoms.
Conclusion
Neurodegenerative disorders are on the rise, and traditional approaches focused on treating symptoms have largely failed to stem this growing tide. By viewing these conditions as metabolic icebergs, we gain a deeper understanding of their root causes and open up new avenues for prevention and treatment. Activating mitohormesis through balanced mitochondrial stress and recovery offers a promising path forward. Ultimately, the goal is to address the root causes—restoring energy balance at the cellular level and, in doing so, transforming the lives of those affected by these devastating diseases.
Let’s begin addressing the unseen bulk and base of the iceberg—mitochondrial dysfunction and its environmental drivers—and take a proactive stance toward preventing neurodegenerative disorders. The potential for transformative change is within our reach.
Reference: Phillips, M.C.L., Picard, M. Neurodegenerative disorders, metabolic icebergs, and mitohormesis. Transl Neurodegener 13, 46 (2024). https://doi.org/10.1186/s40035-024-00435-8
Dr. Christopher Palmer is a Harvard psychiatrist and researcher working at the interface of metabolism and mental health. He is the Founder and Director of the Metabolic and Mental Health Program and the Director of the Department of Postgraduate and Continuing Education at McLean Hospital and an Assistant Professor of Psychiatry at Harvard Medical School. For almost 30 years, he has held administrative, educational, research, and clinical roles in psychiatry at McLean and Harvard. He has been pioneering the use of the medical ketogenic diet in the treatment of psychiatric disorders—conducting research in this area, treating patients, writing, and speaking around the world on this topic. Most recently, he has proposed that mental disorders can be understood as metabolic disorders affecting the brain, which has received widespread recognition in both national and international media outlets.


